How Does Hydrogen Fuel Cell Work
Hydrogen fuel cells are becoming a technology with a high potential to offer significant energy efficiency and decarbonization benefits to various industries. Some industries that have or could benefit include the heavy transport and automotive industry. So, how does a hydrogen fuel cell work?
The professionals in the energy sector have been working on and improving hydrogen fuel cell technology in the search for an emission-free source of energy. The main aim is to provide an alternative to the traditional combustion process and achieves total decarbonization.
A hydrogen fuel cell is among the technologies with much potential for the medium and long term. This is because the fuel cells allow Hydrogen to be stored as an energy source.
Though not available readily in the environment, Hydrogen is always a part of some other substance. Therefore, it is among the most abundant sources of energy. However, a chemical conversion process should be carried out from substances such as water or oil to get this new kind of fuel.
However, this post won’t place a lot of focus on the conversion process to obtain Hydrogen. Rather, it will discuss the application of this element as an energy source for the fuel.
You will learn the benefits of hydrogen fuel cells and get the answer that boggles most people; how does hydrogen fuel cell work? But before then;
What Is A Hydrogen Fuel Cell?
To understand how hydrogen fuel cells work, it is important to understand what a fuel cell is. A fuel cell is an electrochemical device that converts a fuel’s chemical energy and some oxidizing agent into electrical energy. This all happens thanks to reduction-oxidation reactions, a chemical reaction that usually changes the oxidation state of an atom.
A fuel cell and a battery are similar because both generate electrical energy. However, the battery will store the energy in sulfuric acid or lithium solution, whereas a fuel cell uses internal sources like Hydrogen. Hence, the fuel cell can continue operating so long as enough fuel is available.
Therefore, a hydrogen fuel cell is a fuel cell that uses Hydrogen’s chemical energy to produce electrical energy. The energy produced by a hydrogen fuel cell is clean and only has water and heat as the only by-products and, of course, only the energy as the product.
There are many applications of hydrogen fuel cells, from emergency backup power to transportation. It also can power large systems to the size of a power plant or small gadgets such as a laptop.
As we shall see later, fuel cells offer many advantages over traditional combustion-based technologies, such as lower emissions and greater efficiencies.
Because the only by-products of a hydrogen fuel cell are heat and water, they contribute toward decarbonization and reducing the effects of global warming. They also don’t have any moving parts, meaning they operate silently.
How Does Hydrogen Fuel Cell Work?
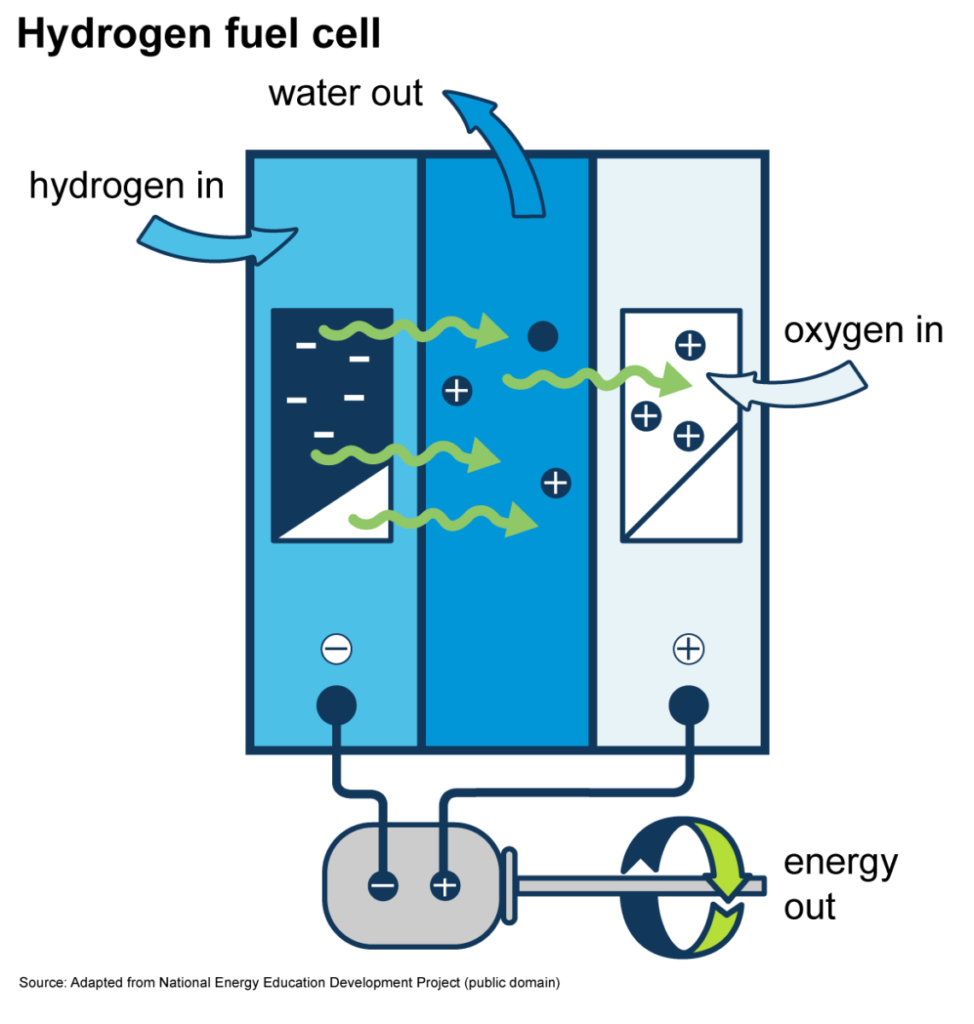
A hydrogen fuel cell uses a chemical/electrochemical reaction to generate electrical power. Each fuel cell consists of two electrodes, the positive electrode (cathode) and the negative electrode (anode). The reaction that produces electrical energy happens at these electrodes.
An electrolyte usually carries the electrically charged particles between the two electrodes. However, a hydrogen fuel cell relies on a catalyst, normally Platinum, to speed up the reaction. Platinum is preferred because it has a higher heat tolerance, meaning that it can withstand higher temperatures compared to other metals. The catalyst also separates the Hydrogen into electrons and protons. This generates the electrical current.
The basic fuel in this fuel cell is Hydrogen. However, the cell also requires oxygen for it to work. Though there are other fuel cells, this is the cleanest and most efficient.
The other kinds of fuel cells include hydrocarbons such as biogas, methanol, or even natural gas. Using an electrochemical process instead of the traditional combustion, the hydrogen fuel cell is highly efficient.
Further, the heat generated by the unit can be used for cooling and heating purposes. So, How Does Hydrogen Fuel Cell Work? The process through which a hydrogen fuel cell generates electrical energy can be summarized as below:
1. First, the Hydrogen stored in reformers or tanks is fed into the fuel cell’s anode using compressors, while the oxygen into the air is fed into the fuel cell through the cathode.
2. The hydrogen molecules on the negative electrode are split into protons and electrons.
3. The protons (positively charged hydrogen ions) pass through an electrolyte or a membrane to the positive electrode. The electrons take a different path as they’re forced into a circuit to generate electrical energy.
4. After flowing through the membrane and circuit accordingly, the protons and electrons met at the positive electrode. They combine with oxygen and produce water and heat as the only by-products.
Because the fuel cells don’t generate a huge amount of electrical energy, they are stacked up to generate enough power for the intended application, whether to power a small gadget or a huge power plant.
A hydrogen fuel cell is highly reliable. Now that you have the answer to how hydrogen fuel cells work, can it be applied to cars, and is there a future for hydrogen-powered cars?
Is there a Future for Hydrogen Fuel Cell Cars?
Many manufacturers have heavily invested in this technology, with some creating hydrogen fuel cell for cars in small numbers. But could hydrogen-powered cars meet the future needs of the transport industry?
Hydrogen has in the past been used for powering engines and is one of the most abundant elements, if not the most, on earth. The ability to produce high amounts of power in a small device signifies that hydrogen-fueled cars can travel longer distances than all-electric cars.
There only needs to be a powerful hydrogen fuel cell for cars. Such vehicles won’t pollute the environment seeing that the only by-products are heat and water.
There is a high demand for clean transport. For instance, battery-powered electric vehicle sales rose by 162% by November 2020. However, this also means that most investors and manufacturers invest in EVs instead of Hydrogen. The lack of infrastructure is the other barrier to the uptake of hydrogen-powered vehicles.
The hydrogen fuel stations for drivers are too few compared to diesel or diesel stations. This means that Hydrogen for fuel cell cars is limited in stations, further complicating the adoption of these vehicles.
Despite all the challenges above, there are several reasons why hydrogen fuels may be the future of the vehicle or car industry. Some of the reasons are the fuelling time, abundance of Hydrogen for fuel cell cars, and the numerous environmental benefits,
Some manufacturers are already investing in or considering hydrogen fuel cells for cars as a complementary electrical power source. Such vehicles are currently producing around the same levels of carbon over a vehicle’s lifetime (120g/km for the hydrogen fuel cells and 124g/km for the EVs).
However, using biomass to get the Hydrogen for fuel cell cars may see the lifecycle emissions from the fuel cell cars dropping to about 60g/km of carbon. This is a considerably lower amount than what could be achieved by EVs.
However, to really come into fruition and truly be the future of the vehicle, there need to be considered investments in hydrogen fuel cells for the cars. This means investing in the technology itself and its supporting infrastructure that could allow readily-available refueling. Unless this happens, the hydrogen-powered cars will not be able to compete with the petrol, diesel, or EV-powered cars.
Benefits of Hydrogen Fuel Cell Technology
Readily Available and Renewable
As aforementioned, Hydrogen has the highest abundance of all the elements on the earth. Despite the associated challenges of extracting it from water, it is a highly abundant renewable energy source. It is the perfect solution to the zero-carbon source of energy.
Flexible and Clean Source of Energy That Is In Line With Decarbonization Efforts
The hydrogen fuel cell technology offers a clean energy source that doesn’t impact the environment since its by-products are only water and heat. Additionally, Hydrogen does not need a large land surface to produce, unlike hydropower and biofuel. Hydrogen’s use, production, and storage will further help in renewable energy development.
Higher Efficiency Compared To Other Sources of Energy
The hydrogen fuels have higher efficiency than the other energy sources, including the environmentally friendly energy solutions. This fuel efficiency ensures a higher amount of energy/pound of fuel. For instance, the traditional combustion-based power plants produce electrical power at an efficiency of 33-35% compared to the hydrogen fuels, which are about 65%.
Fast Charging Times
Hydrogen fuel cell powering units have an extremely rapid charge time similar to the internal combustion engine vehicles. They are also markedly faster compared to battery-powered EVs. The EV might take about 30-60 minutes to charge fully, so a hydrogen fuel cell-powered car can take under 5 minutes to recharge fully. Hence, hydrogen fuel cell cars will have similar flexibility as conventional vehicles.
Conclusion
With the world leaders and investors looking for ways of decarbonization and green energy, hydrogen fuel cells are one solution that surely has a lot of benefits.
Having been used for several decades for various applications, large or small and with the high abundance of Hydrogen and being clean energy, it is time for further research and investment into this technology. From aviation, industrial, transport, and hydrogen fuel cell for cars, the potential for this energy is unlimited.
Recent Posts
Understanding Energy and Electricity: The Power For Progress
Energy and Electricity Energy and electricity are integral components of modern life, powering everything from homes and businesses to transportation and communication. Without them, the...
The Future of Wind Energy The future of wind energy is set to play a critical role in addressing global energy needs while combating climate change. As renewable energy sources like wind and...


